You blow up a spherical balloon to a diameter of 50.0 cm until the absolute pressure inside is 1.25 atm and the temperature is 22.0 C. Assume that all the gas is N_2, of molar mass 28.0 g/mol.
a) Find the mass of a single N_2 molecule using the above information and Avagadro's Number.
b) How much translational kinetic energy does an average N_2 molecule have?
c) How many N_2 molecules are in this balloon?
d) What is the total translational kinetic energy of all the molecules in the balloon?
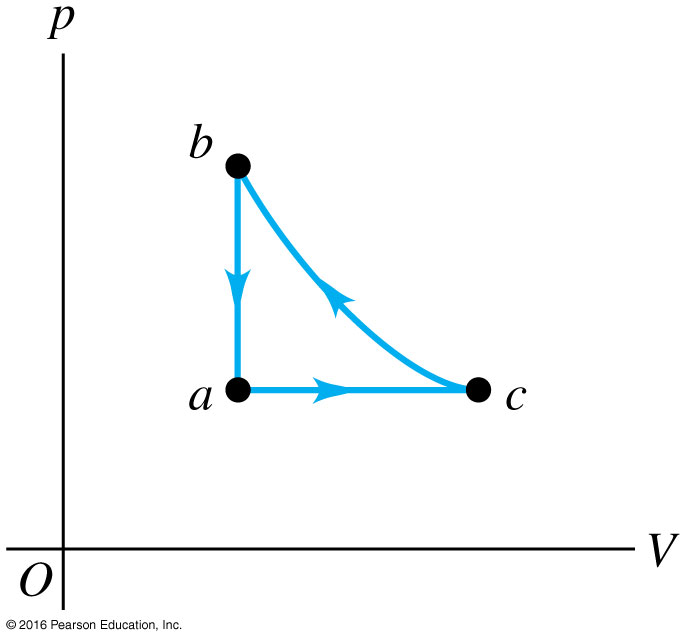
Three moles of an ideal gas are taken around cycle acb. For this gas, C_P=29.1 J/mol/K. Process ac is at constant pressure, process ba is at constant volume, and process cb is adiabatic. The temperatures of the gas in states a, c, and b are T_a=300 K, T_c=492 K, and T_b=600 K. Calculate the total work W for the cycle.
What is the thermal efficiency of an engine that operates by taking n moles of diatomic ideal gas through the cycle 1-->2-->3-->4-->1?