1. The work done during loop I is
A. Positive B. Negative C. Zero D. Infinite E. Indeterminate
2. The work done during loop II is
A. Positive B. Negative C. Zero D. Infinite E. Indeterminate
3. The magnitude of the work done in loop I is
A. greater than the magnitude of work done in II B. less than the magnitude of work done in II C. the same as the magnitude of work done in II D. zero
4. The net work done over both loops is
A. Positive B. Negative C. Zero D. Infinite E. Indeterminate
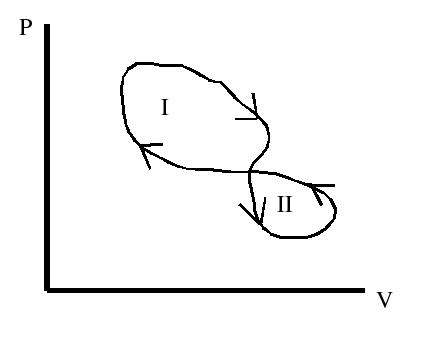
5. Over one complete cycle (loops I and II) the temperature change is
A. Positive B. Negative C. Zero D. Infinite E. Indeterminate
6. During loop I the heat added to the system is
A. greater than zero B. less than zero (in other words heat is removed from the system) C. zero D. could be any of the above
7. During loop II the heat added to the system is
A. greater than zero B. less than zero (in other words heat is removed from the system) C. zero D. could be any of the above
8. Over both loops the total heat added to the system is
A. greater than zero B. less than zero (in other words heat is removed from the system) C. zero D. could be any of the above