27
Which
levels will be occupied?
•The higher the temperature, the higher the
typical level
–Collisions can knock electrons to higher
levels,
if moving atoms have enough kinetic energy
if moving atoms have enough kinetic energy
–At T ~ 300 K (room
T) almost all H in ground state
(n=1)
–At T ~ 10,000 K many H are in first
excited state (n=2)
–At T ~ 15,000 K many H are ionized
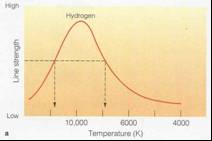
•Because you have highest n=2 population
at ~10,000K
you also have highest Balmer line strength there.
you also have highest Balmer line strength there.
•This gives us another way to estimate
temperatures of stars

From our text: Horizons, by Seeds
n=1
n=2
n=3