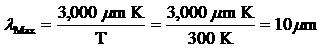1
Astr
1050 Homework 5
Question 1: ALL
statements are true, Kirchoff’s Laws and Balmer Lines.
a. If light comprising a
continuous spectrum passes through a cool low-density gas, the result will be an absorption spectrum.
b. The first three lines
of the Balmer series are visible to human eyes.
c. A solid, liquid, or
dense gas excited to emit light will radiate at all wavelengths and thus produce a continuous spectrum.
d.Transitions from the
first three levels of Hydrogen -- the Lyman, Balmer, and Paschen series -- occur at ultraviolet, optical, and
infrared wavelengths.
e. A low-density gas
excited to emit light will do so at specific wavelengths and thus produce an emission spectrum.
Question 2: According to Wien's
law, an object at room temperature (approximately 300K)
will radiate the most energy at a wavelength of
a.0.5 microns
b.5 microns
c.10 microns
d.100 microns.
e. 5 millimeters