Atoms –
Electron Configuration
•Molecules: Multiple
atoms sharing/exchanging electrons (H2O, CH4)
•Ions: Single atoms where one or more
electrons have escaped (H+)
•Binding
energy: Energy needed to let electron escape
•Permitted
“orbits” or energy levels
–From quantum mechanics, only certain “orbits” are
allowed
–Ground
State: Atom with electron in lowest energy
orbit
–Excited
State: Atom with at least one atom in a higher
energy orbit
–Transition: As electron jumps from one energy level
orbit to another,
atom must release/absorb energy different, usually as light.
atom must release/absorb energy different, usually as light.
•Because only certain orbits are allowed, only certain
energy jumps are allowed,
and atoms can absorb or
emit only certain energies (wavelengths) of light.
•In complicated molecules or “solids” many transitions
are allowed
•Can use
energy levels to “fingerprint” elements
and estimate temperatures.
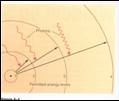
From our text: Horizons, by Seeds