|
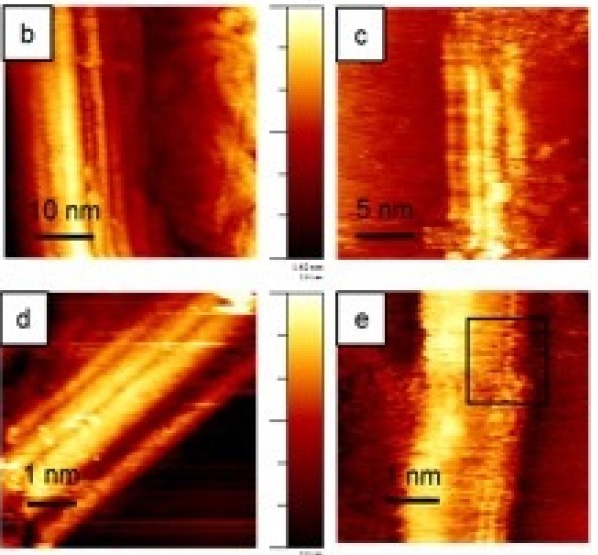
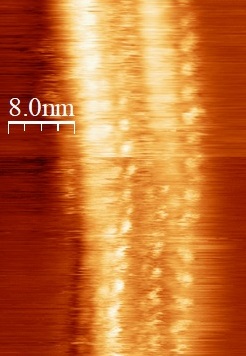
Modified Carbon Nanotubes as Efficient Oxygen Reduction Reaction CatalystsThe current state-of-the-art oxygen reduction reaction (ORR) catalysis is carbon supported platinum nanoparticles. Though the efficiency is high, the cost of the catalysts are not economically vaible for commercial use. Thus, searching for inexpensive materials, such as precious-metal-free catalysts, is the main focus here. In this work, a method of modifying multiwalled carbon nanotubes (MWCNT) with defects and carbonyl groups are presented. These modifications are confirmed to give rise to highly stable and efficient ORR catalytic activities.Read more: J. Stacy et al., ChemistrySelect 2, 1932-1938 (2017). |
|
Catalytic CO2 Capture via Ultrasonically Activating Dually Functionalized Carbon NanotubesHigh energy consumption and high cost have been the obstacles for large-scale deployment of all state-of-the-art CO2 capture technologies. Finding a transformational way to improve mass transfer and reaction kinetics of the CO2 capture process is timely for reducing carbon footprints. In this work, commercial single-walled carbon nanotubes (CNTs) were activated with nitric acid and urea under ultrasonication and hydrothermal methods, respectively, to prepare N-doped CNTs with the functional group of -COOH, which possesses both basic and acid functionalities. The chemically modified CNTs with a concentration of 300 ppm universally catalyze both CO2 sorption and desorption of the CO2 capture process. The increases in the desorption rate achieved with the chemically modified CNTs can reach as high as 503% compared to that of the sorbent without the catalyst. A chemical mechanism underlying the catalytic CO2 capture is proposed based on the experimental results and further confirmed by density functional theory computations. Read more: Y. Gao et al., ACS Nano, 17, 8345-8354 (2023). |